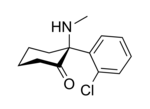
Ketamine
Ketamine is a dissociative anesthetic used medically for induction and maintenance of anesthesia. It is also used as a treatment for depression and in pain management.[19] Ketamine is an NMDA receptor antagonist which accounts for most of its psychoactive effects.[20]
At anesthetic doses, ketamine induces a state of dissociative anesthesia, a trance-like state providing pain relief, sedation, and amnesia.[21] Its distinguishing features as an anesthestic are preserved breathing and airway reflexes, stimulated heart function with increased blood pressure, and moderate bronchodilation.[21] At lower, sub-anesthetic doses, it is a promising agent for treatment of pain and treatment-resistant depression.[22] As with many antidepressants, the results of a single administration wane with time.[23]
Ketamine is used as a recreational drug for its hallucinogenic and dissociative effects.[24] When used recreationally, it is found both in crystalline powder and liquid form, and is often referred to by users as "Special K" or simply "K". The long-term effects of repeated use are largely unknown and are an area of active investigation.[25][26][27] Liver and urinary toxicity have been reported among regular users of high doses of ketamine for recreational purposes.[28]
Ketamine was first synthesized in 1962, derived from phencyclidine in pursuit of a safer anesthetic with fewer hallucinogenic effects.[29][30] It was approved for use in the United States in 1970.[19] It has been regularly used in veterinary medicine and was extensively used for surgical anesthesia in the Vietnam War.[31] It is on the World Health Organization's List of Essential Medicines.[32] It is available as a generic medication.[33]
Medical uses
[edit]Anesthesia
[edit]The use of ketamine in anesthesia reflects its characteristics. It is a drug of choice for short-term procedures when muscle relaxation is not required.[34] The effect of ketamine on the respiratory and circulatory systems is different from that of other anesthetics. It suppresses breathing much less than most other available anesthetics.[35] When used at anesthetic doses, ketamine usually stimulates rather than depresses the circulatory system.[36] Protective airway reflexes are preserved,[37] and it is sometimes possible to administer ketamine anesthesia without protective measures to the airways.[34] Psychotomimetic effects limit the acceptance of ketamine; however, lamotrigine[38] and nimodipine[39] decrease psychotomimetic effects and can also be counteracted by benzodiazepines or propofol administration.[40] Ketofol is a combination of ketamine and propofol.
Ketamine is frequently used in severely injured people and appears to be safe in this group.[41] It has been widely used for emergency surgery in field conditions in war zones,[42] for example, during the Vietnam War.[43] A 2011 clinical practice guideline supports the use of ketamine as a sedative in emergency medicine, including during physically painful procedures.[21] It is the drug of choice for people in traumatic shock who are at risk of hypotension.[44] Ketamine is unlikely to lower blood pressure, which is dangerous for people with severe head injury;[45] in fact, it can raise blood pressure, often making it useful in treating such injuries.[46][47]
Ketamine is an option in children as the sole anesthetic for minor procedures or as an induction agent followed by neuromuscular blocker and tracheal intubation.[42] In particular, children with cyanotic heart disease and neuromuscular disorders are good candidates for ketamine anesthesia.[40][48]
Due to the bronchodilating properties of ketamine, it can be used for anesthesia in people with asthma, chronic obstructive airway disease, and with severe reactive airway disease including active bronchospasm.[42][40][49]
Pain
[edit]Ketamine infusions are used for acute pain treatment in emergency departments and in the perioperative period for individuals with refractory pain. The doses are lower than those used for anesthesia, usually referred to as sub-anesthetic doses. Adjunctive to morphine or on its own, ketamine reduces morphine use, pain level, nausea, and vomiting after surgery. Ketamine is likely to be most beneficial for surgical patients when severe post-operative pain is expected, and for opioid-tolerant patients.[50][51]
Ketamine is especially useful in the pre-hospital setting due to its effectiveness and low risk of respiratory depression.[52] Ketamine has similar efficacy to opioids in a hospital emergency department setting for the management of acute pain and the control of procedural pain.[53] It may also prevent opioid-induced hyperalgesia[54][55] and postanesthetic shivering.[56]
For chronic pain, ketamine is used as an intravenous analgesic, mainly if the pain is neuropathic.[30] It has the added benefit of counteracting spinal sensitization or wind-up phenomena experienced with chronic pain.[57] In multiple clinical trials, ketamine infusions delivered short-term pain relief in neuropathic pain diagnoses, pain after a traumatic spine injury, fibromyalgia, and complex regional pain syndrome (CRPS).[30] However, the 2018 consensus guidelines on chronic pain concluded that, overall, there is only weak evidence in favor of ketamine use in spinal injury pain, moderate evidence in favor of ketamine for CRPS, and weak or no evidence for ketamine in mixed neuropathic pain, fibromyalgia, and cancer pain. In particular, only for CRPS, there is evidence of medium to longer-term pain relief.[30]
Depression
[edit]Ketamine is a rapid-acting antidepressant,[19] but its effect is transient.[58] Intravenous ketamine infusion in treatment-resistant depression may result in improved mood within 4 hours reaching the peak at 24 hours.[22][25] A single dose of intravenous ketamine has been shown to result in a response rate greater than 60% as early as 4.5 hours after the dose (with a sustained effect after 24 hours) and greater than 40% after 7 days.[59] Although only a few pilot studies have sought to determine the optimal dose, increasing evidence suggests that 0.5 mg/kg dose injected over 40 minutes gives an optimal outcome.[60] The antidepressant effect of ketamine is diminished at 7 days, and most people relapse within 10 days. However, for a significant minority, the improvement may last 30 days or more.[25][26][59][61]
One of the main challenges with ketamine treatment can be the length of time that the antidepressant effects last after finishing a course of treatment. A possible option may be maintenance therapy with ketamine, which usually runs twice a week to once in two weeks.[25][26][27] Ketamine may decrease suicidal thoughts for up to three days after the injection.[62]
An enantiomer of ketamine – esketamine commercially sold as Spravato – was approved as an antidepressant by the European Medicines Agency in 2019.[63] Esketamine was approved as a nasal spray for treatment-resistant depression in the United States[64] and elsewhere in 2019 (see Esketamine and Depression). The Canadian Network for Mood and Anxiety Treatments (CANMAT) recommends esketamine as a third-line treatment for depression.[26]
A Cochrane review of randomized controlled trials in adults with unipolar major depressive disorder,[19] found that when compared with placebo, people treated with either ketamine or esketamine experienced reduction or remission of symptoms lasting 1 to 7 days.[65] There were 18.7% (4.1 to 40.4%) more people reporting some benefit and 9.6% (0.2 to 39.4%) more who achieved remission within 24 hours of ketamine treatment. Among people receiving esketamine, 2.1% (2.5 to 24.4%) encountered some relief at 24 hours, and 10.3% (4.5 to 18.2%) had few or no symptoms. These effects did not persist beyond one week, although a higher dropout rate in some studies means that the benefit duration remains unclear.[65]
Ketamine may partially improve depressive symptoms[19] among people with bipolar depression at 24 hours after treatment, but not three or more days.[66] Potentially, ten more people with bipolar depression per 1000 may experience brief improvement, but not the cessation of symptoms, one day following treatment. These estimates are based on limited available research.[66]
In February 2022, the US Food and Drug Administration issued an alert to healthcare professionals concerning compounded nasal spray products containing ketamine intended to treat depression.[67]
Seizures
[edit]Ketamine is used to treat status epilepticus[68] that has not responded to standard treatments, but only case studies and no randomized controlled trials support its use.[69][70]
Asthma
[edit]Ketamine has been suggested as a possible therapy for children with severe acute asthma who do not respond to standard treatment.[71] This is due to its bronchodilator effects.[71] A 2012 Cochrane review found there were minimal adverse effects reported, but the limited studies showed no significant benefit.[71]
Contraindications
[edit]Some major contraindications for ketamine are:[30][50]
- Severe cardiovascular disease such as unstable angina or poorly controlled hypertension
- Increased intracranial or intraocular pressure (however these remain controversial, with recent studies suggesting otherwise)[50]
- Poorly controlled psychosis
- Severe liver disease such as cirrhosis
- Pregnancy
- Active substance use disorder (for serial ketamine injections)
- Age less than 3 months[10]
Adverse effects
[edit]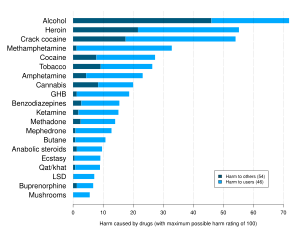
At anesthetic doses, 10–20% of adults and 1–2% of children[10] experience adverse psychiatric reactions that occur during emergence from anesthesia, ranging from dreams and dysphoria to hallucinations and emergence delirium.[73] Psychotomimetic effects decrease adding lamotrigine[38] and nimodipine[39] and can be counteracted by pretreatment with a benzodiazepine or propofol.[73][40] Ketamine anesthesia commonly causes tonic-clonic movements (greater than 10% of people) and rarely hypertonia.[14][73] Vomiting can be expected in 5–15% of the patients; pretreatment with propofol mitigates it as well.[10][73] Laryngospasm occurs only rarely with ketamine. Ketamine, generally, stimulates breathing; however, in the first 2–3 minutes of a high-dose rapid intravenous injection, it may cause a transient respiratory depression.[73]
At lower sub-anesthetic doses, psychiatric side effects are prominent. Most people feel strange, spacey, woozy, or a sense of floating, or have visual distortions or numbness. Also very frequent (20–50%) are difficulty speaking, confusion, euphoria, drowsiness, and difficulty concentrating.[citation needed] The symptoms of psychosis such as going into a hole, disappearing, feeling as if melting, experiencing colors, and hallucinations are described by 6–10% of people. Dizziness, blurred vision, dry mouth, hypertension, nausea, increased or decreased body temperature, or feeling flushed are the common (>10%) non-psychiatric side effects. All these adverse effects are most pronounced by the end of the injection, dramatically reduced 40 minutes afterward, and completely disappear within 4 hours after the injection.[74]
Urinary and liver toxicity
[edit]Urinary toxicity occurs primarily in people who use large amounts of ketamine routinely, with 20–30% of frequent users having bladder complaints.[30][75] It includes a range of disorders from cystitis to hydronephrosis to kidney failure.[76] The typical symptoms of ketamine-induced cystitis are frequent urination, dysuria, and urinary urgency sometimes accompanied by pain during urination and blood in urine.[77] The damage to the bladder wall has similarities to both interstitial and eosinophilic cystitis. The wall is thickened and the functional bladder capacity is as low as 10–150 mL.[76] Studies indicate that ketamine-induced cystitis is caused by ketamine and its metabolites directly interacting with urothelium, resulting in damage of the epithelial cells of the bladder lining and increased permeability of the urothelial barrier which results in clinical symptoms.[78]
Management of ketamine-induced cystitis involves ketamine cessation as the first step. This is followed by NSAIDs and anticholinergics and, if the response is insufficient, by tramadol. The second line treatments are epithelium-protective agents such as oral pentosan polysulfate or intravesical (intra-bladder) instillation of hyaluronic acid. Intravesical botulinum toxin is also useful.[76]
Liver toxicity of ketamine involves higher doses and repeated administration. In a group of chronic high-dose ketamine users, the frequency of liver injury was reported to be about 10%.[citation needed] There are case reports of increased liver enzymes involving ketamine treatment of chronic pain.[76] Chronic ketamine abuse has also been associated with biliary colic,[79] cachexia, gastrointestinal diseases, hepatobiliary disorder, and acute kidney injury.[80]
Near-death experience
[edit]Most people who were able to remember their dreams during ketamine anesthesia report near-death experiences (NDEs) when the broadest possible definition of an NDE is used.[81] Ketamine can reproduce features that commonly have been associated with NDEs.[82] A 2019 large-scale study found that written reports of ketamine experiences had a high degree of similarity to written reports of NDEs in comparison to other written reports of drug experiences.[83]
Dependence and tolerance
[edit]Although the incidence of ketamine dependence is unknown, some people who regularly use ketamine develop ketamine dependence. Animal experiments also confirm the risk of misuse.[24] Additionally, the rapid onset of effects following insufflation may increase potential use as a recreational drug. The short duration of effects promotes bingeing. Ketamine tolerance rapidly develops, even with repeated medical use, prompting the use of higher doses. Some daily users reported withdrawal symptoms, primarily anxiety, shaking, sweating, and palpitations, following the attempts to stop.[24] Cognitive deficits as well as increased dissociation and delusion symptoms were observed in frequent recreational users of ketamine.[84]
Interactions
[edit]Ketamine potentiates the sedative effects of propofol[85] and midazolam.[86] Naltrexone potentiates psychotomimetic effects of a low dose of ketamine,[87] while lamotrigine[38] and nimodipine[39] decrease them. Clonidine reduces the increase of salivation, heart rate, and blood pressure during ketamine anesthesia and decreases the incidence of nightmares.[88]
Clinical observations suggest that benzodiazepines may diminish the antidepressant effects of ketamine.[89] It appears most conventional antidepressants can be safely combined with ketamine.[89]
Pharmacology
[edit]Pharmacodynamics
[edit]Mechanism of action
[edit]Ketamine is a mixture of equal amounts of two enantiomers: esketamine and arketamine. Esketamine is a far more potent NMDA receptor pore blocker than arketamine.[11] Pure blocking of the NMDA receptor is responsible for the anesthetic, analgesic, and psychotomimetic effects of ketamine.[20][90] Blocking of the NMDA receptor results in analgesia by preventing central sensitization in dorsal horn neurons; in other words, ketamine's actions interfere with pain transmission in the spinal cord.[14]
The mechanism of action of ketamine in alleviating depression is not well understood and is an area of active investigation. Due to the hypothesis that NMDA receptor antagonism underlies the antidepressant effects of ketamine, esketamine was developed as an antidepressant.[11] However, multiple other NMDA receptor antagonists, including memantine, lanicemine, rislenemdaz, rapastinel, and 4-chlorokynurenine, have thus far failed to demonstrate significant effectiveness for depression.[11][91] Furthermore, animal research indicates that arketamine, the enantiomer with a weaker NMDA receptor antagonism, as well as (2R,6R)-hydroxynorketamine, the metabolite with negligible affinity for the NMDA receptor but potent alpha-7 nicotinic receptor antagonist activity, may have antidepressant action.[11][92] This furthers the argument that NMDA receptor antagonism may not be primarily responsible for the antidepressant effects of ketamine.[11][93][91] Acute inhibition of the lateral habenula, a part of the brain responsible for inhibiting the mesolimbic reward pathway and referred to as the "anti-reward center", is another possible mechanism for ketamine's antidepressant effects.[94][95][96]
Possible biochemical mechanisms of ketamine's antidepressant action include direct action on the NMDA receptor and downstream effects on regulators such as BDNF and mTOR.[94] It is not clear whether ketamine alone is sufficient for antidepressant action or its metabolites are also important; the active metabolite of ketamine, hydroxynorketamine, which does not significantly interact with the NMDA receptor but nonetheless indirectly activates AMPA receptors, may also or alternatively be involved in the rapid-onset antidepressant effects of ketamine.[20][94][97] In NMDA receptor antagonism, acute blockade of NMDA receptors in the brain results in an increase in the release of glutamate, which leads to an activation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPA receptors), which in turn modulate a variety of downstream signaling pathways to influence neurotransmission in the limbic system and mediate antidepressant effects.[59][94][98] Such downstream actions of the activation of AMPA receptors include upregulation of brain-derived neurotrophic factor (BDNF) and activation of its signaling receptor tropomyosin receptor kinase B (TrkB), activation of the mammalian target of rapamycin (mTOR) pathway, deactivation of glycogen synthase kinase 3 (GSK-3), and inhibition of the phosphorylation of the eukaryotic elongation factor 2 (eEF2) kinase.[59][94][99][100]
Molecular targets
[edit]| Site | Value (μM) | Type | Action | Species | Ref |
|---|---|---|---|---|---|
| NMDA | 0.25–0.66 | Ki | Antagonist | Human | [101][102] |
| MOR | 42 | Ki | Antagonist | Human | [103] |
| MOR2 | 12.1 | Ki | Antagonist | Human | [104] |
| KOR | 28 25 |
Ki Ki |
Antagonist Agonist |
Human | [103] [105] |
| σ2 | 26 | Ki | ND | Rat | [106] |
| D2 | 0.5 >10 |
Ki Ki |
Agonist ND |
Human | [107] [102][108][109] |
| M1 | 45 | Ki | ND | Human | [110] |
| α2β2 | 92 | IC50 | Antagonist | Human | [111] |
| α2β4 | 29 | IC50 | Antagonist | Human | [111] |
| α3β2 | 50 | IC50 | Antagonist | Human | [111] |
| α3β4 | 9.5 | IC50 | Antagonist | Human | [111] |
| α4β2 | 72 | IC50 | Antagonist | Human | [111] |
| α4β4 | 18 | IC50 | Antagonist | Human | [111] |
| α7 | 3.1 (HNK) | IC50 | NAM | Rat | [92] |
| ERα | 0.34 | Ki | ND | Human | [112] |
| NET | 82–291 | IC50 | Inhibitor | Human | [113][114] |
| DAT | 63 | Ki | Inhibitor | Rat | [113] |
| HCN1 | 8–16 | EC50 | Inhibitor | Mouse | [115] |
| TRPV1 | 1-100 | Ki | Agonist | Rat | [116] |
| The smaller the value, the stronger the interaction with the site. | |||||
Ketamine principally acts as a pore blocker of the NMDA receptor, an ionotropic glutamate receptor.[117] The S-(+) and R-(–) stereoisomers of ketamine bind to the dizocilpine site of the NMDA receptor with different affinities, the former showing approximately 3- to 4-fold greater affinity for the receptor than the latter. As a result, the S isomer is a more potent anesthetic and analgesic than its R counterpart.[118]
Ketamine may interact with and inhibit the NMDAR via another allosteric site on the receptor.[119]
With a couple of exceptions, ketamine actions at other receptors are far weaker than ketamine's antagonism of the NMDA receptor (see the activity table to the right).[7][120]
Although ketamine is a very weak ligand of the monoamine transporters (Ki > 60 μM), it has been suggested that it may interact with allosteric sites on the monoamine transporters to produce monoamine reuptake inhibition.[102] However, no functional inhibition (IC50) of the human monoamine transporters has been observed with ketamine or its metabolites at concentrations of up to 10,000 nM.[108][117] Moreover, animal studies and at least three human case reports have found no interaction between ketamine and the monoamine oxidase inhibitor (MAOI) tranylcypromine, which is of importance as the combination of a monoamine reuptake inhibitor with an MAOI can produce severe toxicity such as serotonin syndrome or hypertensive crisis.[121][122] Collectively, these findings shed doubt on the involvement of monoamine reuptake inhibition in the effects of ketamine in humans.[121][117][108][122] Ketamine has been found to increase dopaminergic neurotransmission in the brain, but instead of being due to dopamine reuptake inhibition, this may be via indirect/downstream mechanisms, namely through antagonism of the NMDA receptor.[117][108]
Whether ketamine is an agonist of D2 receptors is controversial. Early research by the Philip Seeman group found ketamine to be a D2 partial agonist with a potency similar to that of its NMDA receptor antagonism.[107][123][124] However, later studies by different researchers found the affinity of ketamine of >10 μM for the regular human and rat D2 receptors,[102][108][109] Moreover, whereas D2 receptor agonists such as bromocriptine can rapidly and powerfully suppress prolactin secretion,[125] subanesthetic doses of ketamine have not been found to do this in humans and in fact, have been found to dose-dependently increase prolactin levels.[126][127] Imaging studies have shown mixed results on inhibition of striatal [11C] raclopride binding by ketamine in humans, with some studies finding a significant decrease and others finding no such effect.[128] However, changes in [11C] raclopride binding may be due to changes in dopamine concentrations induced by ketamine rather than binding of ketamine to the D2 receptor.[128]
Relationships between levels and effects
[edit]Dissociation and psychotomimetic effects are reported in people treated with ketamine at plasma concentrations of approximately 100 to 250 ng/mL (0.42–1.1 μM).[20] The typical intravenous antidepressant dosage of ketamine used to treat depression is low and results in maximal plasma concentrations of 70 to 200 ng/mL (0.29–0.84 μM).[58] At similar plasma concentrations (70 to 160 ng/mL; 0.29–0.67 μM) it also shows analgesic effects.[58] In 1–5 minutes after inducing anesthesia by rapid intravenous injection of ketamine, its plasma concentration reaches as high as 60–110 μM.[129][130] When the anesthesia was maintained using nitrous oxide together with continuous injection of ketamine, the ketamine concentration stabilized at approximately 9.3 μM.[129] In an experiment with purely ketamine anesthesia, people began to awaken once the plasma level of ketamine decreased to about 2,600 ng/mL (11 μM) and became oriented in place and time when the level was down to 1,000 ng/mL (4 μM).[131] In a single-case study, the concentration of ketamine in cerebrospinal fluid, a proxy for the brain concentration, during anesthesia varied between 2.8 and 6.5 μM and was approximately 40% lower than in plasma.[132]
Pharmacokinetics
[edit]Ketamine can be absorbed by many different routes due to both its water and lipid solubility. Intravenous ketamine bioavailability is 100% by definition, intramuscular injection bioavailability is slightly lower at 93%,[7] and epidural bioavailability is 77%.[9] Subcutaneous bioavailability has never been measured but is presumed to be high.[133] Among the less invasive routes, the intranasal route has the highest bioavailability (45–50%)[7][10] and oral – the lowest (16–20%).[7][10] Sublingual and rectal bioavailabilities are intermediate at approximately 25–50%.[7][11][10]
After absorption ketamine is rapidly distributed into the brain and other tissues.[90] The plasma protein binding of ketamine is variable at 23–47%.[12]

In the body, ketamine undergoes extensive metabolism. It is biotransformed by CYP3A4 and CYP2B6 isoenzymes into norketamine, which, in turn, is converted by CYP2A6 and CYP2B6 into hydroxynorketamine and dehydronorketamine.[20] Low oral bioavailability of ketamine is due to the first-pass effect and, possibly, ketamine intestinal metabolism by CYP3A4.[17] As a result, norketamine plasma levels are several-fold higher than ketamine following oral administration, and norketamine may play a role in anesthetic and analgesic action of oral ketamine.[7][17] This also explains why oral ketamine levels are independent of CYP2B6 activity, unlike subcutaneous ketamine levels.[17][134]
After an intravenous injection of tritium-labelled ketamine, 91% of the radioactivity is recovered from urine and 3% from feces.[15] The medication is excreted mostly in the form of metabolites, with only 2% remaining unchanged. Conjugated hydroxylated derivatives of ketamine (80%) followed by dehydronorketamine (16%) are the most prevalent metabolites detected in urine.[31]
Chemistry
[edit]Structure
[edit]In chemical structure, ketamine is an arylcyclohexylamine derivative. Ketamine is a chiral compound. The more active enantiomer, esketamine (S-ketamine), is also available for medical use under the brand name Ketanest S,[135] while the less active enantiomer, arketamine (R-ketamine), has never been marketed as an enantiopure drug for clinical use. While S-ketamine is more effective as an analgesic and anesthetic through NMDA receptor antagonism, R-ketamine produces longer-lasting effects as an antidepressant.[19]
The optical rotation of a given enantiomer of ketamine can vary between its salts and free base form. The free base form of (S)‑ketamine exhibits dextrorotation and is therefore labelled (S)‑(+)‑ketamine. However, its hydrochloride salt shows levorotation and is thus labelled (S)‑(−)‑ketamine hydrochloride.[136]
Detection
[edit]Ketamine may be quantitated in blood or plasma to confirm a diagnosis of poisoning in hospitalized people, provide evidence in an impaired driving arrest, or assist in a medicolegal death investigation. Blood or plasma ketamine concentrations are usually in a range of 0.5–5.0 mg/L in persons receiving the drug therapeutically (during general anesthesia), 1–2 mg/L in those arrested for impaired driving, and 3–20 mg/L in victims of acute fatal overdosage. Urine is often the preferred specimen for routine drug use monitoring purposes. The presence of norketamine, a pharmacologically active metabolite, is useful for confirmation of ketamine ingestion.[137][138][139]
History
[edit]Ketamine was first synthesized in 1962 by Calvin L. Stevens,[19] a professor of chemistry at Wayne State University and a Parke-Davis consultant. It was known by the developmental code name CI-581.[19] After promising preclinical research in animals, ketamine was tested in human prisoners in 1964.[31] These investigations demonstrated ketamine's short duration of action and reduced behavioral toxicity made it a favorable choice over phencyclidine (PCP) as an anesthetic.[140] The researchers wanted to call the state of ketamine anesthesia "dreaming", but Parke-Davis did not approve of the name. Hearing about this problem and the "disconnected" appearance of treated people, Mrs. Edward F. Domino,[141] the wife of one of the pharmacologists working on ketamine, suggested "dissociative anesthesia".[31] Following FDA approval in 1970, ketamine anesthesia was first given to American soldiers during the Vietnam War.[142]
The discovery of antidepressive action of ketamine in 2000[143] has been described as the single most important advance in the treatment of depression in more than 50 years.[61][11] It has sparked interest in NMDA receptor antagonists for depression,[144] and has shifted the direction of antidepressant research and development.[145]
Society and culture
[edit]Legal status
[edit]While ketamine is marketed legally in many countries worldwide,[146] it is also a controlled substance in many countries.[7]
- In Australia, ketamine is listed as a Schedule 8 controlled drug under the Poisons Standard (October 2015).[147]
- In Canada, ketamine has been classified as a Schedule I narcotic, since 2005.[148]
- In December 2013, the government of India, in response to rising recreational use and the use of ketamine as a date rape drug, added it to Schedule X of the Drug and Cosmetics Act requiring a special license for sale and maintenance of records of all sales for two years.[149][150]
- In the United Kingdom, it became labeled a Class B drug on 12 February 2014.[151][152]
- The increase in recreational use prompted ketamine to be placed in Schedule III of the United States Controlled Substances Act in August 1999.[153]
Recreational use
[edit]At sub-anesthetic doses, ketamine produces a dissociative state, characterised by a sense of detachment from one's physical body and the external world that is known as depersonalization and derealization.[154] At sufficiently high doses, users may experience what is called the "K-hole", a state of dissociation with visual and auditory hallucination.[155] John C. Lilly, Marcia Moore, D. M. Turner, and David Woodard (among others) have written extensively about their own entheogenic and psychonautic experiences with ketamine.[156] Turner died prematurely due to drowning during presumed unsupervised ketamine use.[157] In 2006, the Russian edition of Adam Parfrey's Apocalypse Culture was banned and destroyed by authorities owing to its inclusion of an essay by Woodard about the entheogenic use of, and psychonautic experiences with, ketamine.[158]: 288–295
Recreational ketamine use has been implicated in deaths globally, with more than 90 deaths in England and Wales in the years of 2005–2013.[159] They include accidental poisonings, drownings, traffic accidents, and suicides.[159] The majority of deaths were among young people.[160] Several months after being found dead in his hot tub, actor Matthew Perry's October 2023 apparent drowning death was revealed to have been caused by a ketamine overdose, and while other factors were present, the acute effects of ketamine were ruled to be the primary cause of death.[161] Due to its ability to cause confusion and amnesia, ketamine has been used for date rape.[162][142]
Research
[edit]Ketamine is under investigation for its potential in treating treatment-resistant depression.[163][164][165] Ketamine is a known psychoplastogen, which refers to a compound capable of promoting rapid and sustained neuroplasticity.[166]
Ketamine has shown anthelmintic activity in rats, with an effect comparable to ivermectin and albendazole at extremely high concentrations.[167]
Veterinary uses
[edit]In veterinary anesthesia, ketamine is often used for its anesthetic and analgesic effects on cats,[168] dogs,[169] rabbits, rats, and other small animals.[170][171] It is frequently used in induction and anesthetic maintenance in horses. It is an important part of the "rodent cocktail", a mixture of drugs used for anesthetising rodents.[172] Veterinarians often use ketamine with sedative drugs to produce balanced anesthesia and analgesia, and as a constant-rate infusion to help prevent pain wind-up. Ketamine is also used to manage pain among large animals. It is the primary intravenous anesthetic agent used in equine surgery, often in conjunction with detomidine and thiopental, or sometimes guaifenesin.[173]
Ketamine appears not to produce sedation or anesthesia in snails. Instead, it appears to have an excitatory effect.[174]
References
[edit]- ^ Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 159–. ISBN 978-94-011-4439-1. Archived from the original on 11 April 2017.
- ^ "Ketamine (Ketalar) Use During Pregnancy". Drugs.com. 22 November 2019. Archived from the original on 26 June 2020. Retrieved 18 May 2020.
- ^ "Drug Scheduling". U.S. Drug Enforcement Administration. Archived from the original on 8 April 2024. Retrieved 29 December 2023. Ketamine is listed in Schedule III.
- ^ Huang, MC., Lin, SK. (2020). "Ketamine Abuse: Past and Present". In: Hashimoto, K., Ide, S., Ikeda, K. (eds.) Ketamine. Springer, Singapore. doi:10.1007/978-981-15-2902-3_1.
- ^ Bell RF, Eccleston C, Kalso EA (June 2017). "Ketamine as an adjuvant to opioids for cancer pain" (PDF). The Cochrane Database of Systematic Reviews. 6 (9): CD003351. doi:10.1002/14651858.CD003351.pub3. PMC 6481583. PMID 28657160. Archived (PDF) from the original on 12 January 2024. Retrieved 10 September 2018.
- ^ Moyse DW, Kaye AD, Diaz JH, Qadri MY, Lindsay D, Pyati S (March 2017). "Perioperative Ketamine Administration for Thoracotomy Pain". Pain Physician. 20 (3): 173–184. PMID 28339431.
- ^ a b c d e f g h i j k l m n o Mathew SJ, Zarate Jr CA (25 November 2016). Ketamine for Treatment-Resistant Depression: The First Decade of Progress. Springer. pp. 8–10, 14–22. ISBN 978-3-319-42925-0. Archived from the original on 8 September 2017.
- ^ Brayfield A, ed. (9 January 2017). "Ketamine Hydrochloride: Martindale: The Complete Drug Reference". MedicinesComplete. London, UK: Pharmaceutical Press. Archived from the original on 28 August 2021. Retrieved 24 August 2017.
- ^ a b Kintz P (22 March 2014). Toxicological Aspects of Drug-Facilitated Crimes. Elsevier Science. pp. 87–. ISBN 978-0-12-416969-2. Archived from the original on 8 September 2017.
- ^ a b c d e f g h i Marland S, Ellerton J, Andolfatto G, Strapazzon G, Thomassen O, Brandner B, et al. (June 2013). "Ketamine: use in anesthesia". CNS Neurosci Ther. 19 (6): 381–9. doi:10.1111/cns.12072. PMC 6493613. PMID 23521979.
- ^ a b c d e f g h Hashimoto K (October 2019). "Rapid-acting antidepressant ketamine, its metabolites and other candidates: A historical overview and future perspective". Psychiatry and Clinical Neurosciences. 73 (10): 613–627. doi:10.1111/pcn.12902. PMC 6851782. PMID 31215725.
- ^ a b Dayton PG, Stiller RL, Cook DR, Perel JM (1983). "The binding of ketamine to plasma proteins: emphasis on human plasma". Eur J Clin Pharmacol. 24 (6): 825–31. doi:10.1007/BF00607095. PMID 6884418. S2CID 807011.
- ^ a b c d e f g h Sinner B, Graf BM (2008). "Ketamine". In Schüttler J, Schwilden H (eds.). Modern Anesthetics. Handbook of Experimental Pharmacology. Vol. 182. pp. 313–33. doi:10.1007/978-3-540-74806-9_15. ISBN 978-3-540-72813-9. PMID 18175098.
- ^ a b c d e f g h Quibell R, Prommer EE, Mihalyo M, Twycross R, Wilcock A (March 2011). "Ketamine*". Journal of Pain and Symptom Management (Therapeutic Review). 41 (3): 640–9. doi:10.1016/j.jpainsymman.2011.01.001. PMID 21419322. Archived from the original on 16 September 2018. Retrieved 28 July 2014.
- ^ a b c Chang T, Glazko AJ (1974). "Biotransformation and disposition of ketamine". Int Anesthesiol Clin. 12 (2): 157–77. doi:10.1097/00004311-197412020-00018. PMID 4603048. S2CID 30723730.
- ^ Hijazi Y, Boulieu R (July 2002). "Contribution of CYP3A4, CYP2B6, and CYP2C9 isoforms to N-demethylation of ketamine in human liver microsomes". Drug Metabolism and Disposition. 30 (7): 853–8. doi:10.1124/dmd.30.7.853. PMID 12065445. S2CID 15787750.
- ^ a b c d Rao LK, Flaker AM, Friedel CC, Kharasch ED (December 2016). "Role of Cytochrome P4502B6 Polymorphisms in Ketamine Metabolism and Clearance". Anesthesiology. 125 (6): 1103–1112. doi:10.1097/ALN.0000000000001392. PMID 27763887. S2CID 41380105.
- ^ Sass W, Fusari S (1977). "Ketamine". Analytical Profiles of Drug Substances. Vol. 6. Academic Press. pp. 297–322. doi:10.1016/S0099-5428(08)60347-0. ISBN 9780122608063.
- ^ a b c d e f g h Sachdeva B, Sachdeva P, Ghosh S, Ahmad F, Sinha JK (March 2023). "Ketamine as a therapeutic agent in major depressive disorder and posttraumatic stress disorder: Potential medicinal and deleterious effects". Ibrain. 9 (1): 90–101. doi:10.1002/ibra.12094. ISSN 2769-2795. PMC 10528797. PMID 37786516. S2CID 257117630.
- ^ a b c d e f Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, et al. (July 2018). "Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms". Pharmacol Rev. 70 (3): 621–660. doi:10.1124/pr.117.015198. PMC 6020109. PMID 29945898.
- ^ a b c Green SM, Roback MG, Kennedy RM, Krauss B (May 2011). "Clinical practice guideline for emergency department ketamine dissociative sedation: 2011 update". Annals of Emergency Medicine. 57 (5): 449–461. doi:10.1016/j.annemergmed.2010.11.030. PMID 21256625.
- ^ a b Zhang K, Hashimoto K (January 2019). "An update on ketamine and its two enantiomers as rapid-acting antidepressants". Expert Review of Neurotherapeutics. 19 (1): 83–92. doi:10.1080/14737175.2019.1554434. PMID 30513009. S2CID 54628949.
- ^ Hibicke M, Landry AN, Kramer HM, Talman ZK, Nichols CD (March 2020). "Psychedelics, but Not Ketamine, Produce Persistent Antidepressant-like Effects in a Rodent Experimental System for the Study of Depression". ACS Chemical Neuroscience. 11 (6): 864–871. doi:10.1021/acschemneuro.9b00493. PMID 32133835. S2CID 212418003.
- ^ a b c Morgan CJ, Curran HV (January 2012). "Ketamine use: a review". Addiction. 107 (1): 27–38. doi:10.1111/j.1360-0443.2011.03576.x. PMID 21777321. S2CID 11064759.
- ^ a b c d Marcantoni WS, Akoumba BS, Wassef M, Mayrand J, Lai H, Richard-Devantoy S, et al. (December 2020). "A systematic review and meta-analysis of the efficacy of intravenous ketamine infusion for treatment resistant depression: January 2009 – January 2019". J Affect Disord. 277: 831–841. doi:10.1016/j.jad.2020.09.007. PMID 33065824. S2CID 223557698.
- ^ a b c d Swainson J, McGirr A, Blier P, Brietzke E, Richard-Devantoy S, Ravindran N, et al. (November 2020). "The Canadian Network for Mood and Anxiety Treatments (CANMAT) Task Force Recommendations for the Use of Racemic Ketamine in Adults with Major Depressive Disorder: Recommandations Du Groupe De Travail Du Réseau Canadien Pour Les Traitements De L'humeur Et De L'anxiété (Canmat) Concernant L'utilisation De La Kétamine Racémique Chez Les Adultes Souffrant De Trouble Dépressif Majeur". Can J Psychiatry. 66 (2): 113–125. doi:10.1177/0706743720970860. PMC 7918868. PMID 33174760.
- ^ a b Bobo WV, Riva-Posse P, Goes FS, Parikh SV (April 2020). "Next-Step Treatment Considerations for Patients With Treatment-Resistant Depression That Responds to Low-Dose Intravenous Ketamine". Focus (Am Psychiatr Publ). 18 (2): 181–192. doi:10.1176/appi.focus.20190048. PMC 7587874. PMID 33162856.
- ^ Orhurhu VJ, Vashisht R, Claus LE, Cohen SP (April 2022). "Ketamine toxicity". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 31082131. Archived from the original on 16 May 2022. Retrieved 18 August 2022.
- ^ Peltoniemi MA, Hagelberg NM, Olkkola KT, Saari TI (September 2016). "Ketamine: A Review of Clinical Pharmacokinetics and Pharmacodynamics in Anesthesia and Pain Therapy". Clinical Pharmacokinetics. 55 (9): 1059–77. doi:10.1007/s40262-016-0383-6. PMID 27028535. S2CID 5078489.
- ^ a b c d e f Cohen SP, Bhatia A, Buvanendran A, Schwenk ES, Wasan AD, Hurley RW, et al. (July 2018). "Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Chronic Pain From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists". Reg Anesth Pain Med. 43 (5): 521–546. doi:10.1097/AAP.0000000000000808. PMC 6023575. PMID 29870458.
- ^ a b c d Domino EF (September 2010). "Taming the ketamine tiger. 1965". Anesthesiology. 113 (3): 678–84. doi:10.1097/ALN.0b013e3181ed09a2. PMID 20693870.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ "Ketamine Injection". Drugs.com. Archived from the original on 10 December 2014. Retrieved 1 December 2014.
- ^ a b Rosenbaum SB, Gupta V, Palacios JL (2020). "Ketamine". StatPearls. StatPearls Publishing. PMID 29262083. Archived from the original on 12 November 2020. Retrieved 5 March 2020.
- ^ Heshmati F, Zeinali MB, Noroozinia H, Abbacivash R, Mahoori A (December 2003). "Use of ketamine in severe status asthmaticus in intensive care unit". Iranian Journal of Allergy, Asthma, and Immunology. 2 (4): 175–80. PMID 17301376. Archived from the original on 6 October 2014.
- ^ Adams HA (December 1997). "[S-(+)-ketamine. Circulatory interactions during total intravenous anesthesia and analgesia-sedation]" [S-(+)-ketamine. Circulatory interactions during total intravenous anesthesia and analgesia-sedation]. Der Anaesthesist (in German). 46 (12): 1081–7. doi:10.1007/s001010050510. PMID 9451493. S2CID 36323023.
- ^ Wong JJ, Lee JH, Turner DA, Rehder KJ (August 2014). "A review of the use of adjunctive therapies in severe acute asthma exacerbation in critically ill children". Expert Review of Respiratory Medicine. 8 (4): 423–41. doi:10.1586/17476348.2014.915752. PMID 24993063. S2CID 31435021.
- ^ a b c Anand A, Charney DS, Oren DA, Berman RM, Hu XS, Cappiello A, et al. (March 2000). "Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists". Arch Gen Psychiatry. 57 (3): 270–6. doi:10.1001/archpsyc.57.3.270. PMID 10711913.
- ^ a b c Krupitsky EM, Burakov AM, Romanova TN, Grinenko NI, Grinenko AY, Fletcher J, et al. (December 2001). "Attenuation of ketamine effects by nimodipine pretreatment in recovering ethanol dependent men: psychopharmacologic implications of the interaction of NMDA and L-type calcium channel antagonists". Neuropsychopharmacology. 25 (6): 936–47. doi:10.1016/S0893-133X(01)00346-3. PMID 11750186.
- ^ a b c d Barrett W, Buxhoeveden M, Dhillon S (October 2020). "Ketamine: a versatile tool for anesthesia and analgesia". Current Opinion in Anesthesiology. 33 (5): 633–638. doi:10.1097/ACO.0000000000000916. PMID 32826629. S2CID 221236545.
- ^ Cohen L, Athaide V, Wickham ME, Doyle-Waters MM, Rose NG, Hohl CM (January 2015). "The effect of ketamine on intracranial and cerebral perfusion pressure and health outcomes: a systematic review". Annals of Emergency Medicine. 65 (1): 43–51.e2. doi:10.1016/j.annemergmed.2014.06.018. PMID 25064742.
- ^ a b c Kurdi MS, Theerth KA, Deva RS (September 2014). "Ketamine: Current applications in anesthesia, pain, and critical care". Anesthesia: Essays and Researches. 8 (3): 283–90. doi:10.4103/0259-1162.143110. PMC 4258981. PMID 25886322.
- ^ Mion G (September 2017). "History of anaesthesia: The ketamine story – past, present and future". Eur J Anaesthesiol. 34 (9): 571–575. doi:10.1097/EJA.0000000000000638. PMID 28731926. S2CID 27536846.
- ^ Nickson C (7 August 2013). "Intubation, Hypotension and Shock". Life in the Fastlane (blog). Critical Care Compendium. Archived from the original on 9 February 2014. Retrieved 10 April 2014.[unreliable medical source?]
- ^ Manley G, Knudson MM, Morabito D, Damron S, Erickson V, Pitts L (October 2001). "Hypotension, hypoxia, and head injury: frequency, duration, and consequences". Archives of Surgery. 136 (10): 1118–23. doi:10.1001/archsurg.136.10.1118. PMID 11585502.
- ^ Hemmingsen C, Nielsen JE (November 1991). "Intravenous ketamine for prevention of severe hypotension during spinal anaesthesia". Acta Anaesthesiologica Scandinavica. 35 (8): 755–7. doi:10.1111/j.1399-6576.1991.tb03385.x. PMID 1763596. S2CID 1324453.
- ^ Wong DH, Jenkins LC (May 1975). "The cardiovascular effects of ketamine in hypotensive states". Canadian Anaesthetists' Society Journal. 22 (3): 339–48. doi:10.1007/BF03004843. PMID 1139377.
- ^ Bali A, Dang AK, Gonzalez DA, Kumar R, Asif S (24 April 2024). "Clinical Uses of Ketamine in Children: A Narrative Review - PMC". Cureus. 14 (7): e27065. doi:10.7759/cureus.27065. PMC 9389002. PMID 35989801.
- ^ Goyal S, Agrawal A (May 2013). "Ketamine in status asthmaticus: A review". Indian Journal of Critical Care Medicine. 17 (3): 154–61. doi:10.4103/0972-5229.117048. PMC 3777369. PMID 24082612.
- ^ a b c Schwenk ES, Viscusi ER, Buvanendran A, Hurley RW, Wasan AD, Narouze S, et al. (July 2018). "Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Acute Pain Management From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists". Reg Anesth Pain Med. 43 (5): 456–466. doi:10.1097/AAP.0000000000000806. PMC 6023582. PMID 29870457.
- ^ Sin B, Ternas T, Motov SM (March 2015). "The use of subdissociative-dose ketamine for acute pain in the emergency department". Academic Emergency Medicine. 22 (3): 251–7. doi:10.1111/acem.12604. PMID 25716117. S2CID 24658476.
- ^ Svenson J, Biedermann M (2011). "Ketamine: a unique drug with several potential uses in the prehospital setting". Journal of Paramedic Practice. 3 (10): 552–556. doi:10.12968/jpar.2011.3.10.552.
- ^ Karlow N, Schlaepfer CH, Stoll CR, Doering M, Carpenter CR, Colditz GA, et al. (October 2018). "A Systematic Review and Meta-analysis of Ketamine as an Alternative to Opioids for Acute Pain in the Emergency Department". Academic Emergency Medicine. 25 (10): 1086–1097. doi:10.1111/acem.13502. PMID 30019434.
- ^ Radvansky BM, Shah K, Parikh A, Sifonios AN, Le V, Eloy JD (2015). "Role of ketamine in acute postoperative pain management: a narrative review". BioMed Research International. 2015: 749837. doi:10.1155/2015/749837. PMC 4606413. PMID 26495312.
- ^ Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L (2011). "A comprehensive review of opioid-induced hyperalgesia". Pain Physician. 14 (2): 145–61. doi:10.36076/ppj.2011/14/145. PMID 21412369.
- ^ Zhou Y, Mannan A, Han Y, Liu H, Guan HL, Gao X, et al. (December 2019). "Efficacy and safety of prophylactic use of ketamine for prevention of postanesthetic shivering: a systematic review and meta analysis". BMC Anesthesiology. 19 (1): 245. doi:10.1186/s12871-019-0910-8. PMC 6937868. PMID 31888509.
- ^ Elia N, Tramèr MR (January 2005). "Ketamine and postoperative pain—a quantitative systematic review of randomised trials". Pain. 113 (1–2): 61–70. doi:10.1016/j.pain.2004.09.036. PMID 15621365. S2CID 25925720.
- ^ a b c Sanacora G, Frye MA, McDonald W, Mathew SJ, Turner MS, Schatzberg AF, et al. (April 2017). "A Consensus Statement on the Use of Ketamine in the Treatment of Mood Disorders". JAMA Psychiatry. 74 (4): 399–405. doi:10.1001/jamapsychiatry.2017.0080. PMID 28249076. S2CID 28320520.
- ^ a b c d Molero P, Ramos-Quiroga JA, Martin-Santos R, Calvo-Sánchez E, Gutiérrez-Rojas L, Meana JJ (May 2018). "Antidepressant Efficacy and Tolerability of Ketamine and Esketamine: A Critical Review". CNS Drugs. 32 (5): 411–420. doi:10.1007/s40263-018-0519-3. PMID 29736744. S2CID 13679905.
- ^ Sanacora G, Katz R (July 2018). "Ketamine: A Review for Clinicians". Focus. 16 (3). American Psychiatric Association Publishing: 243–250. doi:10.1176/appi.focus.20180012. PMC 6493090. PMID 31975918.
- ^ a b Singh I, Morgan C, Curran V, Nutt D, Schlag A, McShane R (May 2017). "Ketamine treatment for depression: opportunities for clinical innovation and ethical foresight". The Lancet. Psychiatry. 4 (5): 419–426. doi:10.1016/S2215-0366(17)30102-5. hdl:10871/30208. PMID 28395988. S2CID 28186580. Archived from the original on 9 March 2019. Retrieved 10 September 2018.
- ^ Witt K, Potts J, Hubers A, Grunebaum MF, Murrough JW, Loo C, et al. (January 2020). "Ketamine for suicidal ideation in adults with psychiatric disorders: A systematic review and meta-analysis of treatment trials". Aust N Z J Psychiatry. 54 (1): 29–45. doi:10.1177/0004867419883341. hdl:1959.4/unsworks_76229. PMID 31729893. S2CID 208035394. Archived from the original on 2 July 2022. Retrieved 18 January 2021.
- ^ "Spravato (esketamine)". European Medicines Agency. 8 July 2022. Archived from the original on 23 November 2020. Retrieved 20 July 2022.
- ^ "FDA approves new nasal spray medication for treatment-resistant depression; available only at a certified doctor's office or clinic". US Food and Drug Administration. 5 March 2019. Archived from the original on 23 July 2021. Retrieved 29 July 2022.
- ^ a b Dean RL, Hurducas C, Hawton K, Spyridi S, Cowen PJ, Hollingsworth S, et al. (September 2021). "Ketamine and other glutamate receptor modulators for depression in adults with unipolar major depressive disorder". The Cochrane Database of Systematic Reviews. 9 (11): CD011612. doi:10.1002/14651858.CD011612.pub3. PMC 8434915. PMID 34510411.
- ^ a b Dean RL, Marquardt T, Hurducas C, Spyridi S, Barnes A, Smith R, et al. (October 2021). "Ketamine and other glutamate receptor modulators for depression in adults with bipolar disorder". The Cochrane Database of Systematic Reviews. 2021 (10): CD011611. doi:10.1002/14651858.CD011611.pub3. PMC 8499740. PMID 34623633.
- ^ "FDA alerts health care professionals of potential risks associated with compounded ketamine nasal spray". US Food and Drug Administration. 16 February 2022. Archived from the original on 31 August 2022. Retrieved 29 July 2022.
- ^ Ghosh S, Sinha JK, Khan T, Devaraju KS, Singh P, Vaibhav K, et al. (April 2021). "Pharmacological and Therapeutic Approaches in the Treatment of Epilepsy". Biomedicines. 9 (5): 470. doi:10.3390/biomedicines9050470. PMC 8146518. PMID 33923061.
- ^ Gomes D, Pimentel J, Bentes C, Aguiar de Sousa D, Antunes AP, Alvarez A, et al. (October 2018). "Consensus Protocol for the Treatment of Super-Refractory Status Epilepticus". Acta Médica Portuguesa. 31 (10): 598–605. doi:10.20344/amp.9679. PMID 30387431. Archived from the original on 29 August 2020. Retrieved 11 February 2020.
- ^ Rosati A, De Masi S, Guerrini R (November 2018). "Ketamine for Refractory Status Epilepticus: A Systematic Review". CNS Drugs. 32 (11): 997–1009. doi:10.1007/s40263-018-0569-6. PMID 30232735. S2CID 52302073.
- ^ a b c Jat KR, Chawla D, et al. (Cochrane Airways Group) (November 2012). "Ketamine for management of acute exacerbations of asthma in children". The Cochrane Database of Systematic Reviews. 11 (11): CD009293. doi:10.1002/14651858.CD009293.pub2. PMC 6483733. PMID 23152273.
- ^ Nutt DJ, King LA, Phillips LD (November 2010). "Drug harms in the UK: a multicriteria decision analysis". Lancet. 376 (9752): 1558–1565. CiteSeerX 10.1.1.690.1283. doi:10.1016/S0140-6736(10)61462-6. PMID 21036393. S2CID 5667719.
- ^ a b c d e Strayer RJ, Nelson LS (November 2008). "Adverse events associated with ketamine for procedural sedation in adults". The American Journal of Emergency Medicine. 26 (9): 985–1028. doi:10.1016/j.ajem.2007.12.005. PMID 19091264. Archived from the original on 8 September 2017.
- ^ Acevedo-Diaz EE, Cavanaugh GW, Greenstein D, Kraus C, Kadriu B, Zarate CA, et al. (February 2020). "Comprehensive assessment of side effects associated with a single dose of ketamine in treatment-resistant depression". J Affect Disord. 263: 568–575. doi:10.1016/j.jad.2019.11.028. PMC 8457026. PMID 31791675.
- ^ Smith HS (2010). "Ketamine-induced urologic insult (KIUI)". Pain Physician. 13 (6): E343–6. doi:10.36076/ppj.2010/13/E343. PMID 21102971.
- ^ a b c d Castellani D, Pirola GM, Gubbiotti M, Rubilotta E, Gudaru K, Gregori A, et al. (April 2020). "What urologists need to know about ketamine-induced uropathy: A systematic review". Neurourol Urodyn. 39 (4): 1049–1062. doi:10.1002/nau.24341. PMID 32212278. S2CID 214643776.
- ^ Middela S, Pearce I (January 2011). "Ketamine-induced vesicopathy: a literature review". International Journal of Clinical Practice. 65 (1): 27–30. doi:10.1111/j.1742-1241.2010.02502.x. PMID 21155941. S2CID 25034266. Archived from the original on 19 September 2018. Retrieved 10 September 2018.
- ^ Qixin D, Tianpeng W, Xiaochun Y, Lingqi L, Jiantao Y, Zhongjie L (20 January 2017). "Changes to the bladder epithelial barrier are associated with ketamine-induced cystitis". Experimental and Therapeutic Medicine. 14 (4): 2757–2762. doi:10.3892/etm.2017.4913. PMC 5615221. PMID 28966667.
- ^ Ahamed AN, Yahya AA (2 June 2016). "Chronic biliary colic associated with ketamine abuse". International Medical Case Reports Journal. 9: 135–137. doi:10.2147/IMCRJ.S100648. PMC 4898409. PMID 27330331.
- ^ Joseph P, Binu R, Sebastian T, Fahmy H (11 December 2017). "Multiorgan Dysfunction Related to Chronic Ketamine Abuse". Baylor University Medical Center Proceedings. 27 (3): 223–225. doi:10.1080/08998280.2014.11929117. PMC 4059572. PMID 24982568.
- ^ Jansen K (2001). Ketamine: Dreams and Realities. Multidisciplinary Association for Psychedelic Studies. p. 122. ISBN 978-0-9660019-3-8.
- ^ Peinkhofer C, Dreier JP, Kondziella D (July 2019). "Semiology and Mechanisms of Near-Death Experiences". Current Neurology and Neuroscience Reports. 19 (9): 62. doi:10.1007/s11910-019-0983-2. PMID 31352520. S2CID 198965307.
- ^ Martial C, Cassol H, Charland-Verville V, Pallavicini C, Sanz C, Zamberlan F, et al. (March 2019). "Neurochemical models of near-death experiences: A large-scale study based on the semantic similarity of written reports". Consciousness and Cognition. 69: 52–69. doi:10.1016/j.concog.2019.01.011. hdl:2268/231971. PMID 30711788. S2CID 73432875.
- ^ Morgan CJ, Muetzelfeldt L, Curran HV (January 2010). "Consequences of chronic ketamine self-administration upon neurocognitive function and psychological wellbeing: a 1-year longitudinal study". Addiction. 105 (1): 121–33. doi:10.1111/j.1360-0443.2009.02761.x. PMID 19919593.
- ^ Hui TW, Short TG, Hong W, Suen T, Gin T, Plummer J (March 1995). "Additive interactions between propofol and ketamine when used for anesthesia induction in female patients". Anesthesiology. 82 (3): 641–8. doi:10.1097/00000542-199503000-00005. PMID 7879932. S2CID 24005549.
- ^ Hong W, Short TG, Hui TW (December 1993). "Hypnotic and anesthetic interactions between ketamine and midazolam in female patients". Anesthesiology. 79 (6): 1227–32. doi:10.1097/00000542-199312000-00013. PMID 8267198. S2CID 12246068.
- ^ Krystal JH, Madonick S, Perry E, Gueorguieva R, Brush L, Wray Y, et al. (August 2006). "Potentiation of low dose ketamine effects by naltrexone: potential implications for the pharmacotherapy of alcoholism". Neuropsychopharmacology. 31 (8): 1793–800. doi:10.1038/sj.npp.1300994. PMID 16395307.
- ^ Handa F, Tanaka M, Nishikawa T, Toyooka H (February 2000). "Effects of oral clonidine premedication on side effects of intravenous ketamine anesthesia: a randomized, double-blind, placebo-controlled study". J Clin Anesth. 12 (1): 19–24. doi:10.1016/s0952-8180(99)00131-2. PMID 10773503.
- ^ a b Andrade C (July 2017). "Ketamine for Depression, 5: Potential Pharmacokinetic and Pharmacodynamic Drug Interactions". The Journal of Clinical Psychiatry. 78 (7): e858–e861. doi:10.4088/JCP.17f11802. PMID 28858450.
- ^ a b Peltoniemi MA, Hagelberg NM, Olkkola KT, Saari TI (September 2016). "Ketamine: A Review of Clinical Pharmacokinetics and Pharmacodynamics in Anesthesia and Pain Therapy". Clin Pharmacokinet. 55 (9): 1059–77. doi:10.1007/s40262-016-0383-6. PMID 27028535. S2CID 5078489.
- ^ a b Garay R, Zarate CA, Cavero I, Kim YK, Charpeaud T, Skolnick P (October 2018). "The development of glutamate-based antidepressants is taking longer than expected". Drug Discovery Today. 23 (10): 1689–1692. doi:10.1016/j.drudis.2018.02.006. PMC 6211562. PMID 29501913.
- ^ a b Moaddel R, Abdrakhmanova G, Kozak J, Jozwiak K, Toll L, Jimenez L, et al. (January 2013). "Sub-anesthetic concentrations of (R,S)-ketamine metabolites inhibit acetylcholine-evoked currents in α7 nicotinic acetylcholine receptors". Eur J Pharmacol. 698 (1–3): 228–34. doi:10.1016/j.ejphar.2012.11.023. PMC 3534778. PMID 23183107.
- ^ "Arketamine – Jiangsu Hengrui Medicine – AdisInsight". Archived from the original on 13 April 2021. Retrieved 13 November 2019.
- ^ a b c d e Zanos P, Gould TD (April 2018). "Mechanisms of ketamine action as an antidepressant". Molecular Psychiatry. 23 (4): 801–811. doi:10.1038/mp.2017.255. PMC 5999402. PMID 29532791.
- ^ Kim D, Cheong E, Shin HS (June 2018). "Overcoming Depression by Inhibition of Neural Burst Firing". Neuron. 98 (5): 878–879. doi:10.1016/j.neuron.2018.05.032. PMID 29879390.
- ^ Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, et al. (February 2018). "Ketamine blocks bursting in the lateral habenula to rapidly relieve depression". Nature. 554 (7692): 317–322. Bibcode:2018Natur.554..317Y. doi:10.1038/nature25509. PMID 29446381. S2CID 3334820.
- ^ Zanos P, Thompson SM, Duman RS, Zarate CA, Gould TD (March 2018). "Convergent Mechanisms Underlying Rapid Antidepressant Action". CNS Drugs. 32 (3): 197–227. doi:10.1007/s40263-018-0492-x. PMC 6005380. PMID 29516301.
- ^ Gilbert JR, Yarrington JS, Wills KE, Nugent AC, Zarate CA (August 2018). "Glutamatergic Signaling Drives Ketamine-Mediated Response in Depression: Evidence from Dynamic Causal Modeling". The International Journal of Neuropsychopharmacology. 21 (8): 740–747. doi:10.1093/ijnp/pyy041. PMC 6070027. PMID 29668918.
- ^ Björkholm C, Monteggia LM (March 2016). "BDNF – a key transducer of antidepressant effects". Neuropharmacology. 102: 72–79. doi:10.1016/j.neuropharm.2015.10.034. PMC 4763983. PMID 26519901.
- ^ Castrén E, Kojima M (January 2017). "Brain-derived neurotrophic factor in mood disorders and antidepressant treatments". Neurobiology of Disease. 97 (Pt B): 119–126. doi:10.1016/j.nbd.2016.07.010. hdl:10138/311483. PMID 27425886. S2CID 644350.
- ^ Morris PJ, Moaddel R, Zanos P, Moore CE, Gould TD, Zarate CA, et al. (September 2017). "Synthesis and N-Methyl-d-aspartate (NMDA) Receptor Activity of Ketamine Metabolites". Organic Letters. 19 (17): 4572–4575. doi:10.1021/acs.orglett.7b02177. PMC 5641405. PMID 28829612.
- ^ a b c d Roth BL, Gibbons S, Arunotayanun W, Huang XP, Setola V, Treble R, et al. (2013). "The ketamine analogue methoxetamine and 3- and 4-methoxy analogues of phencyclidine are high affinity and selective ligands for the glutamate NMDA receptor". PLOS ONE. 8 (3): e59334. Bibcode:2013PLoSO...859334R. doi:10.1371/journal.pone.0059334. PMC 3602154. PMID 23527166.
- ^ a b Hirota K, Okawa H, Appadu BL, Grandy DK, Devi LA, Lambert DG (January 1999). "Stereoselective interaction of ketamine with recombinant mu, kappa, and delta opioid receptors expressed in Chinese hamster ovary cells". Anesthesiology. 90 (1): 174–82. doi:10.1097/00000542-199901000-00023. PMID 9915326.
- ^ Hirota K, Sikand KS, Lambert DG (1999). "Interaction of ketamine with mu2 opioid receptors in SH-SY5Y human neuroblastoma cells". Journal of Anesthesia. 13 (2): 107–9. doi:10.1007/s005400050035. PMID 14530949. S2CID 9322174.
- ^ Nemeth CL, Paine TA, Rittiner JE, Béguin C, Carroll FI, Roth BL, et al. (June 2010). "Role of kappa-opioid receptors in the effects of salvinorin A and ketamine on attention in rats". Psychopharmacology (Berl). 210 (2): 263–74. doi:10.1007/s00213-010-1834-7. PMC 2869248. PMID 20358363.
- ^ Robson MJ, Elliott M, Seminerio MJ, Matsumoto RR (April 2012). "Evaluation of sigma (σ) receptors in the antidepressant-like effects of ketamine in vitro and in vivo". Eur Neuropsychopharmacol. 22 (4): 308–17. doi:10.1016/j.euroneuro.2011.08.002. PMID 21911285. S2CID 24494428.
- ^ a b Kapur S, Seeman P (2002). "NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2)receptors-implications for models of schizophrenia". Molecular Psychiatry. 7 (8): 837–44. doi:10.1038/sj.mp.4001093. PMID 12232776.
- ^ a b c d e Can A, Zanos P, Moaddel R, Kang HJ, Dossou KS, Wainer IW, et al. (October 2016). "Effects of Ketamine and Ketamine Metabolites on Evoked Striatal Dopamine Release, Dopamine Receptors, and Monoamine Transporters". The Journal of Pharmacology and Experimental Therapeutics. 359 (1): 159–70. doi:10.1124/jpet.116.235838. PMC 5034706. PMID 27469513.
- ^ a b Jordan S, Chen R, Fernalld R, Johnson J, Regardie K, Kambayashi J, et al. (July 2006). "In vitro biochemical evidence that the psychotomimetics phencyclidine, ketamine and dizocilpine (MK-801) are inactive at cloned human and rat dopamine D2 receptors". European Journal of Pharmacology. 540 (1–3): 53–6. doi:10.1016/j.ejphar.2006.04.026. PMID 16730695.
- ^ Hirota K, Hashimoto Y, Lambert DG (December 2002). "Interaction of intravenous anesthetics with recombinant human M1-M3 muscarinic receptors expressed in chinese hamster ovary cells". Anesth Analg. 95 (6): 1607–10, table of contents. doi:10.1097/00000539-200212000-00025. PMID 12456425. S2CID 25643394.
- ^ a b c d e f Yamakura T, Chavez-Noriega LE, Harris RA (April 2000). "Subunit-dependent inhibition of human neuronal nicotinic acetylcholine receptors and other ligand-gated ion channels by dissociative anesthetics ketamine and dizocilpine". Anesthesiology. 92 (4): 1144–53. doi:10.1097/00000542-200004000-00033. PMID 10754635. S2CID 23651917.
- ^ Ho MF, Correia C, Ingle JN, Kaddurah-Daouk R, Wang L, Kaufmann SH, et al. (June 2018). "Ketamine and ketamine metabolites as novel estrogen receptor ligands: Induction of cytochrome P450 and AMPA glutamate receptor gene expression". Biochemical Pharmacology. 152: 279–292. doi:10.1016/j.bcp.2018.03.032. PMC 5960634. PMID 29621538.
- ^ a b Nishimura M, Sato K, Okada T, Yoshiya I, Schloss P, Shimada S, et al. (March 1998). "Ketamine inhibits monoamine transporters expressed in human embryonic kidney 293 cells". Anesthesiology. 88 (3): 768–74. doi:10.1097/00000542-199803000-00029. PMID 9523822. S2CID 30159489.
- ^ Zhao Y, Sun L (November 2008). "Antidepressants modulate the in vitro inhibitory effects of propofol and ketamine on norepinephrine and serotonin transporter function". J Clin Neurosci. 15 (11): 1264–9. doi:10.1016/j.jocn.2007.11.007. PMC 2605271. PMID 18815045.
- ^ Chen X, Shu S, Bayliss DA (January 2009). "HCN1 channel subunits are a molecular substrate for hypnotic actions of ketamine". The Journal of Neuroscience. 29 (3): 600–9. doi:10.1523/JNEUROSCI.3481-08.2009. PMC 2744993. PMID 19158287.
- ^ da Costa FL, Pinto MC, Santos DC, Carobin NV, de Jesus IC, Ferreira LA, et al. (December 2020). "Ketamine potentiates TRPV1 receptor signaling in the peripheral nociceptive pathways". Biochemical Pharmacology. 182: 114210. doi:10.1016/j.bcp.2020.114210. PMID 32882205. S2CID 221497233.
- ^ a b c d Tyler MW, Yourish HB, Ionescu DF, Haggarty SJ (June 2017). "Classics in Chemical Neuroscience: Ketamine". ACS Chemical Neuroscience. 8 (6): 1122–1134. doi:10.1021/acschemneuro.7b00074. PMID 28418641.
- ^ Hirota K, Lambert DG (October 1996). "Ketamine: its mechanism(s) of action and unusual clinical uses". British Journal of Anaesthesia. 77 (4): 441–4. doi:10.1093/bja/77.4.441. PMID 8942324.
- ^ Orser BA, Pennefather PS, MacDonald JF (April 1997). "Multiple mechanisms of ketamine blockade of N-methyl-D-aspartate receptors". Anesthesiology. 86 (4): 903–17. doi:10.1097/00000542-199704000-00021. PMID 9105235. S2CID 2164198.
- ^ Lodge D, Mercier MS (September 2015). "Ketamine and phencyclidine: the good, the bad, and the unexpected". British Journal of Pharmacology. 172 (17): 4254–76. doi:10.1111/bph.13222. PMC 4556466. PMID 26075331.
- ^ a b Kraus C, Rabl U, Vanicek T, Carlberg L, Popovic A, Spies M, et al. (March 2017). "Administration of ketamine for unipolar and bipolar depression". International Journal of Psychiatry in Clinical Practice. 21 (1): 2–12. doi:10.1080/13651501.2016.1254802. PMID 28097909. S2CID 35626369.
- ^ a b Bartova L, Vogl SE, Stamenkovic M, Praschak-Rieder N, Naderi-Heiden A, Kasper S, et al. (November 2015). "Combination of intravenous S-ketamine and oral tranylcypromine in treatment-resistant depression: A report of two cases". European Neuropsychopharmacology. 25 (11): 2183–4. doi:10.1016/j.euroneuro.2015.07.021. PMID 26302763. S2CID 39039021.
- ^ Seeman P, Guan HC (November 2008). "Phencyclidine and glutamate agonist LY379268 stimulate dopamine D2High receptors: D2 basis for schizophrenia". Synapse. 62 (11): 819–28. doi:10.1002/syn.20561. PMID 18720422. S2CID 206519749.
- ^ Seeman P, Guan HC, Hirbec H (August 2009). "Dopamine D2High receptors stimulated by phencyclidines, lysergic acid diethylamide, salvinorin A, and modafinil". Synapse. 63 (8): 698–704. doi:10.1002/syn.20647. PMID 19391150. S2CID 17758902.
- ^ The Role of Brain Dopamine. Springer Science & Business Media. 6 December 2012. pp. 23–. ISBN 978-3-642-73897-5.
- ^ Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. (March 1994). "Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses". Archives of General Psychiatry. 51 (3): 199–214. doi:10.1001/archpsyc.1994.03950030035004. PMID 8122957.
- ^ Hergovich N, Singer E, Agneter E, Eichler HG, Graselli U, Simhandl C, et al. (May 2001). "Comparison of the effects of ketamine and memantine on prolactin and cortisol release in men. a randomized, double-blind, placebo-controlled trial". Neuropsychopharmacology. 24 (5): 590–3. doi:10.1016/S0893-133X(00)00194-9. PMID 11282259.
- ^ a b Rabiner EA (May 2007). "Imaging of striatal dopamine release elicited with NMDA antagonists: is there anything there to be seen?". Journal of Psychopharmacology. 21 (3): 253–8. doi:10.1177/0269881107077767. PMID 17591653. S2CID 23776189.
- ^ a b Idvall J, Ahlgren I, Aronsen KR, Stenberg P (December 1979). "Ketamine infusions: pharmacokinetics and clinical effects". Br J Anaesth. 51 (12): 1167–73. doi:10.1093/bja/51.12.1167. PMID 526385.
- ^ Domino EF, Zsigmond EK, Domino LE, Domino KE, Kothary SP, Domino SE (February 1982). "Plasma levels of ketamine and two of its metabolites in surgical patients using a gas chromatographic mass fragmentographic assay". Anesth Analg. 61 (2): 87–92. doi:10.1213/00000539-198202000-00004. PMID 7198883. S2CID 27596215.
- ^ White PF, Schüttler J, Shafer A, Stanski DR, Horai Y, Trevor AJ (February 1985). "Comparative pharmacology of the ketamine isomers. Studies in volunteers". Br J Anaesth. 57 (2): 197–203. doi:10.1093/bja/57.2.197. PMID 3970799.
- ^ Stenberg P, Idvall J (July 1981). "Does ketamine metabolite II exist in vivo?". Br J Anaesth. 53 (7): 778. doi:10.1093/bja/53.7.778. PMID 7248132.
- ^ Mao J (19 April 2016). Opioid-Induced Hyperalgesia. CRC Press. pp. 127–. ISBN 978-1-4200-8900-4. Archived from the original on 8 September 2017.
- ^ Li Y, Jackson KA, Slon B, Hardy JR, Franco M, William L, et al. (August 2015). "CYP2B6*6 allele and age substantially reduce steady-state ketamine clearance in chronic pain patients: impact on adverse effects". Br J Clin Pharmacol. 80 (2): 276–84. doi:10.1111/bcp.12614. PMC 4541975. PMID 25702819.
- ^ Krüger AD (1998). "[Current aspects of using ketamine in childhood]". Anaesthesiologie und Reanimation (in German). 23 (3): 64–71. PMID 9707751.
- ^ Chankvetadze B, Burjanadze N, Breitkreutz J, Bergander K, Bergenthal D, Kataeva O, et al. (2002). "Mechanistic study on the opposite migration order of the enantiomers of ketamine with α- and β-cyclodextrin in capillary electrophoresis". Journal of Separation Science. 25 (15–17): 1155–1166. doi:10.1002/1615-9314(20021101)25:15/17<1155::AID-JSSC1155>3.0.CO;2-M.
- ^ Feng N, Vollenweider FX, Minder EI, Rentsch K, Grampp T, Vonderschmitt DJ. Development of a gas chromatography-mass spectrometry method for determination of ketamine in plasma and its application to human samples. Ther. Drug Monit. 17: 95–100, 1995.
- ^ Parkin MC, Turfus SC, Smith NW, Halket JM, Braithwaite RA, Elliott SP, Osselton MD, Cowan DA, Kicman AT. Detection of ketamine and its metabolites in urine by ultra-high-pressure liquid chromatography-tandem mass spectrometry. J. Chrom. B 876: 137–142, 2008.
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 806–808.
- ^ Corssen G, Domino EF (January–February 1966). "Dissociative anesthesia: further pharmacologic studies and first clinical experience with the phencyclidine derivative CI-581". Anesthesia and Analgesia. 45 (1): 29–40. doi:10.1213/00000539-196601000-00007. PMID 5325977. S2CID 29516392.
- ^ Li L, Vlisides PE (2016). "Ketamine: 50 Years of Modulating the Mind". Frontiers in Human Neuroscience. 10: 612. doi:10.3389/fnhum.2016.00612. PMC 5126726. PMID 27965560.
- ^ a b "Ketamine". Center for Substance Abuse Research (CESAR); University of Maryland, College Park. 29 October 2013. Archived from the original on 12 November 2013. Retrieved 27 July 2014.
- ^ Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. (February 2000). "Antidepressant effects of ketamine in depressed patients". Biol Psychiatry. 47 (4): 351–4. doi:10.1016/s0006-3223(99)00230-9. PMID 10686270. S2CID 43438286.
- ^ Chaffrey J (16 March 2022). "Yale Researchers Study Potential Treatment for Depression in Patients With Parkinson's Disease". NBC Connecticut. Archived from the original on 19 March 2022. Retrieved 19 March 2022.
- ^ Dhir A (January 2017). "Investigational drugs for treating major depressive disorder". Expert Opinion on Investigational Drugs. 26 (1): 9–24. doi:10.1080/13543784.2017.1267727. PMID 27960559. S2CID 45232796.
- ^ Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 584–585. ISBN 978-3-88763-075-1.
- ^ Poisons Standard October 2015 "Poisons Standard". Australian Government. October 2015. Archived from the original on 19 January 2016. Retrieved 6 January 2016.
- ^ Legal status of ketamine in Canada references:
- "Statutes of Canada (S.C.) Controlled Drugs and Substances Act (S.C. 1996 c.19) Schedule I § 14". Justice Laws Website. Government of Canada. 12 June 2014. Archived from the original on 22 November 2013.
- "Order Amending Schedule I to the Controlled Drugs and Substances Act" (PDF). Canada Gazette Part II. Vol. 139, no. 19. 21 September 2005. p. 2130. Archived from the original (PDF) on 8 August 2014. Retrieved 2 August 2014.
- "Status of ketamine under CDSA". Canadian Society of Customs Brokers. 2 May 2005. Archived from the original on 10 August 2014. Retrieved 2 August 2014.
- ^ "Ketamine drug brought under 'Schedule X' to curb abuse". The Times of India. 7 January 2014. Archived from the original on 14 April 2014. Retrieved 2 August 2014.
- ^ Sumitra DR (30 December 2013). "Govt makes notorious 'date rape' drug ketamine harder to buy or sell". The Times of India. Archived from the original on 30 December 2013.
- ^ Baker N (12 February 2014), Response to ACMD recommendation on ketamine (PDF) (Correspondence to Les Iverson [chair of]; Advisory Council on the Misuse of Drugs), Crown copyright; Open Government Licence, archived (PDF) from the original on 28 February 2014, retrieved 21 February 2014.
- ^ Dixon H (12 February 2014). "Party drug ketamine to be upgraded to Class B". The Daily Telegraph. Archived from the original on 9 June 2014. Retrieved 2 August 2014.
- ^ Marshall DR (13 July 1999). "Schedules of Controlled Substances: Placement of Ketamine into Schedule III [21 CFR Part 1308. Final Rule 99-17803]" (PDF). Rules and Regulations. Federal Register. 64 (133): 37673–5. Archived (PDF) from the original on 5 May 2015.
- ^ Giannini AJ, Underwood NA, Condon M (November 2000). "Acute ketamine intoxication treated by haloperidol: a preliminary study". American Journal of Therapeutics. 7 (6): 389–91. doi:10.1097/00045391-200007060-00008. PMID 11304647.
- ^ Giannini AJ (1999). Drug Abuse. Los Angeles: Health Information Press. p. 104. ISBN 978-1-885987-11-2.
- ^ References for recreational use in literature:
- Lilly JC (1997). The Scientist: A Metaphysical Autobiography. Berkeley, CA: Ronin. pp. 144–. ISBN 978-0-914171-72-0.
- Kelly K (2001). The Little Book of Ketamine. Ronin. pp. 23, 40–45, 46–51, ibid. ISBN 978-1-57951-121-0.
- Alltounian HS, Moore M (1978). Journeys Into the Bright World. Rockport, MA: Para Research. ISBN 978-0-914918-12-7.
- Palmer C, Horowitz M (2000). Sisters of the Extreme: Women Writing on the Drug Experience. Inner Traditions. pp. 254–258, ibid. ISBN 978-0-89281-757-3.
- Turner DM (1994). The Essential Psychedelic Guide. San Francisco: Panther Press. ISBN 978-0-9642636-1-1.
- ^ Jansen K (2001). Ketamine: Dreams and Realities. Multidisciplinary Association for Psychedelic Studies. pp. 50, 89. ISBN 978-0-9660019-3-8.
- ^ Woodard D (2000). "The Ketamine Necromance". In Parfrey A (ed.). Apocalypse Culture II. Los Angeles: Feral House. pp. 288–295. Archived from the original on 24 June 2021. Retrieved 18 May 2020.
- ^ a b See Max Daly, 2014, "The Sad Demise of Nancy Lee, One of Britain's Ketamine Casualties," at Vice (online), 23 July 2014, see "The Sad Demise of Nancy Lee, One of Britain's Ketamine Casualties". 23 July 2014. Archived from the original on 7 June 2015. Retrieved 7 June 2015., accessed 7 June 2015.
- ^ "Drug related deaths involving ketamine in England and Wales". A report of the Mortality team, Life Events and Population Sources Division, Office for National Statistics. Government of the United Kingdom. 2013. Archived from the original on 7 June 2015. Retrieved 7 June 2015. and "Deaths Related to Drug Poisoning in England and Wales – Office for National Statistics". Archived from the original on 19 June 2015. Retrieved 7 June 2015., accessed 7 June 2015.
- ^ Bowman E (15 December 2023). "Matthew Perry died from the 'acute effects of ketamine,' autopsy finds". NPR. Archived from the original on 28 December 2023. Retrieved 28 December 2023.
- ^ "Do you know... Ketamine". Knowledge Exchange. Toronto: Centre for Addiction and Mental Health. 2003. Archived from the original on 7 April 2014. Retrieved 27 July 2014.
- ^ Grinspoon P (9 August 2022). "Ketamine for treatment-resistant depression: When and where is it safe?". Harvard Health. Archived from the original on 31 August 2022. Retrieved 6 September 2022.
- ^ "New Hope for Treatment-Resistant Depression: Guessing Right on Ketamine". National Institute of Mental Health (NIMH). 13 August 2019. Archived from the original on 10 September 2022. Retrieved 6 September 2022.
- ^ Pérez-Esparza R (2018). "Ketamine for Treatment-Resistant Depression: a New Advocate". Revista de Investigacion Clinica. 70 (2): 65–67. doi:10.24875/RIC.18002501. PMID 29718013.
- ^ Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. (August 2010). "mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists". Science. 329 (5994): 959–964. Bibcode:2010Sci...329..959L. doi:10.1126/science.1190287. PMC 3116441. PMID 20724638.
- ^ Ferreira SR, Machado AR, Furtado LF, Gomes JH, de Almeida RM, de Oliveira Mendes T, et al. (October 2020). "Ketamine can be produced by Pochonia chlamydosporia: an old molecule and a new anthelmintic?". Parasites & Vectors. 13 (1): 527. doi:10.1186/s13071-020-04402-w. PMC 7574484. PMID 33081837.
- ^ Robertson SA, Taylor PM (October 2004). "Pain management in cats—past, present and future. Part 2. Treatment of pain—clinical pharmacology". Journal of Feline Medicine and Surgery. 6 (5): 321–33. doi:10.1016/j.jfms.2003.10.002. PMC 10822209. PMID 15363764. S2CID 25572412.
- ^ Lamont LA (November 2008). "Adjunctive analgesic therapy in veterinary medicine". The Veterinary Clinics of North America. Small Animal Practice. 38 (6): 1187–203, v. doi:10.1016/j.cvsm.2008.06.002. PMID 18954680.
- ^ Stunkard JA, Miller JC (September 1974). "An outline guide to general anesthesia in exotic species". Veterinary Medicine, Small Animal Clinician. 69 (9): 1181–6. PMID 4604091.
- ^ Riviere JE, Papich MG (2009). Veterinary Pharmacology and Therapeutics. John Wiley & Sons. p. 200. ISBN 978-1-118-68590-7. Archived from the original on 8 February 2023. Retrieved 26 December 2021.
- ^ Standard Operating Procedure No. 1 Anesthesia and Analgesia in Rodents, Washington College, 2012, pp. 1–2, archived from the original on 4 August 2013, retrieved 27 November 2015
- ^ Hubbell JA, Muir WW, Sams RA (November 1980). "Guaifenesin: cardiopulmonary effects and plasma concentrations in horses". American Journal of Veterinary Research. 41 (11): 1751–5. PMID 7212404.
- ^ Woodall AJ, McCrohan CR (December 2000). "Excitatory actions of propofol and ketamine in the snail Lymnaea stagnalis". Comparative Biochemistry and Physiology. Toxicology & Pharmacology. 127 (3): 297–305. doi:10.1016/S0742-8413(00)00155-9. PMID 11246501.
External links
[edit]- Ketamine fact sheet — from the United States DEA, via Archive.org
- Analgesics
- Antidepressants
- Arylcyclohexylamines
- Chemical substances for emergency medicine
- 2-Chlorophenyl compounds
- D2-receptor agonists
- Dissociative drugs
- Drug-facilitated sexual assault
- Drugs developed by Pfizer
- Drugs with unknown mechanisms of action
- Equine medications
- Euphoriants
- Experimental antidepressants
- Experimental hallucinogens
- General anesthetics
- Ketones
- Muscarinic antagonists
- NMDA receptor antagonists
- Nicotinic antagonists
- Opioid modulators
- Opioid receptor positive allosteric modulators
- Psychedelics, dissociatives and deliriants
- Sedatives
- Sigma agonists
- World Health Organization essential medicines
- Veterinary medicine